Introduction
Organ transplantation remains a life-saving intervention for patients with end-stage organ failure. However, challenges such as immune rejection and dependency on lifelong immunosuppression continue to limit its long-term success. Decellularization and recellularization offer a promising bioengineering solution: by stripping donor organs of their immunogenic cells and repopulating the scaffolds with a patient’s own cells, it becomes possible to create immunocompatible organs for transplantation.
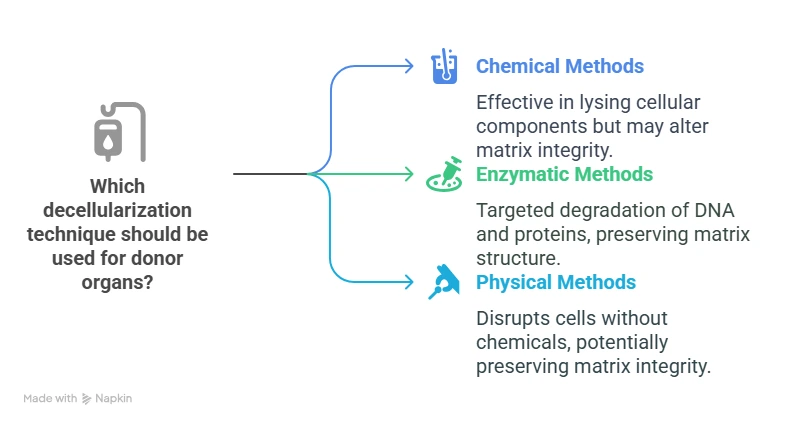
Techniques for Decellularizing Donor Organs
Recellularization Strategies:
These strategies aim to repopulate the scaffold with viable, functional cells to restore organ-specific functions.
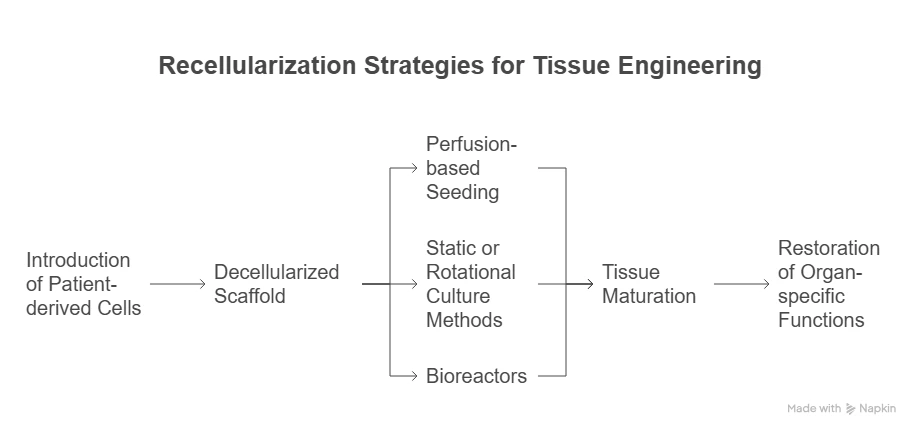
Recellularization involves introducing patient-derived cells—such as induced pluripotent stem cells (iPSCs), mesenchymal stem cells, or organ-specific progenitor cells—into the decellularized scaffold. Techniques include:
Perfusion-based seeding to mimic native vascular delivery routes.
Static or rotational culture methods to facilitate even cell distribution.
Bioreactors for dynamic cultivation and tissue maturation.

Advantages of Recellularized Organs:
By using autologous cells, recellularized organs significantly reduce the risk of immune rejection and eliminate the dependency on immunosuppressants. Additionally, these organs can achieve superior integration and long-term functionality post-transplant, offering new hope for personalized organ replacement therapies.
Clinical Implications and Challenges :
While promising, several hurdles must be addressed for clinical application:
Scalability and reproducibility of decellularization and recellularization protocols.
Functional integration of the regenerated organs in vivo.
Regulatory and ethical considerations, particularly for human trials.
Ongoing research is focused on overcoming these barriers to facilitate broader clinical translation.

