Introduction
Personalized tissue engineering is reshaping regenerative medicine by utilizing cutting-edge imaging technologies and computational models. These tools facilitate the design of patient-specific tissues and organs, ensuring better anatomical fit, enhanced vascularization, and superior integration post-implantation. Recent breakthroughs have paved the way for translating these technologies into clinical applications.
Imaging Techniques in Tissue Engineering:
- MRI (Magnetic Resonance Imaging) and CT (Computed Tomography) scans provide precise anatomical and functional details.
- Confocal microscopy allows visualization at the cellular level. These techniques aid in capturing the microarchitecture and vascular networks essential for engineering functional tissues.
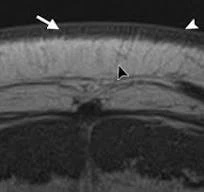
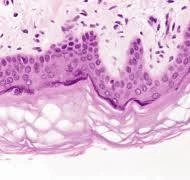
Computational Modeling Approaches: Advanced modeling strategies simulate tissue behavior under physiological conditions, enabling optimized design:
- Finite Element Analysis (FEA): Models mechanical behavior and stress distribution in engineered tissues.
- Agent-Based Modeling: Simulates cellular interactions and tissue growth. These models help predict post-implantation outcomes and personalize constructs based on patient-specific data.
Integration of Imaging and Computational Modeling: Combining imaging data with modeling platforms leads to precise, individualized tissue constructs:
- Imaging-derived geometries guide computational simulations.
- Iterative modeling refines scaffold design for optimal integration and function. This synergistic approach bridges the gap between virtual design and biological reality.
Applications in Tissue Engineering:
Custom-tailored tissues are being developed for a variety of clinical needs:
Challenges and Future Directions:
Despite promising results, challenges remain:
- Integration of multi-modal imaging data into models.
- Standardization and validation of simulation outputs.
- Scalable production methods for custom implants. Future directions include real-time modeling, AI-enhanced simulations, and integration with 3D bioprinting.
Clinical Translation and Regulatory Considerations:
Regulatory pathways must evolve to accommodate personalized constructs:
- Regulatory bodies require robust validation and safety data.
- Ethical considerations focus on data privacy and informed consent. Patient safety and reproducibility are central to successful clinical translation.

