1. Introduction
Tissue engineering, a field that merges materials science and biology, aims to restore or replace damaged tissues. The role of biomaterials in this field is crucial, as they serve as scaffolds to support cell attachment, proliferation, and differentiation. Traditional biomaterials, however, often lack the ability to interact with the dynamic physiological environment, limiting their effectiveness in promoting tissue regeneration. In contrast, responsive biomaterials—engineered to adapt to biological
stimuli—represent a paradigm shift.
2. Background
Initially, biomaterials used in tissue engineering were largely passive—designed to provide structural support without interacting with surrounding tissues. These materials faced challenges such as chronic inflammation, poor integration, and insufficient biodegradability. Over time, researchers sought to create scaffolds that not only support tissue growth but also actively participate in the healing process. This led to the development of bioactive and later, responsive biomaterials that mimic the dynamic nature of the extracellular matrix (ECM).
3. Responsive Biomaterials
3.1 Degradation Dynamics
A critical feature of smart biomaterials is their ability to degrade at a rate that matches new tissue formation. This controlled degradation prevents scaffold accumulation and ensures seamless integration of the newly formed tissue. Examples include:
- Polylactic-co-glycolic acid (PLGA): A widely used biodegradable polymer whose degradation rate can be tuned by adjusting its monomer ratio.
- Silk fibroin: Exhibits enzymatic degradation responsive to the tissue environment.
- Magnesium-based biomaterials: Biodegradable metals with potential in load-bearing orthopedic applications.
The advent of responsive biomaterials marks a significant leap in the field of tissue engineering. By interacting with biological systems in a dynamic and intelligent manner, these materials have the potential to revolutionize regenerative therapies. As research progresses, smart biomaterials are poised to become foundational tools in personalized medicine and advanced tissue repair.
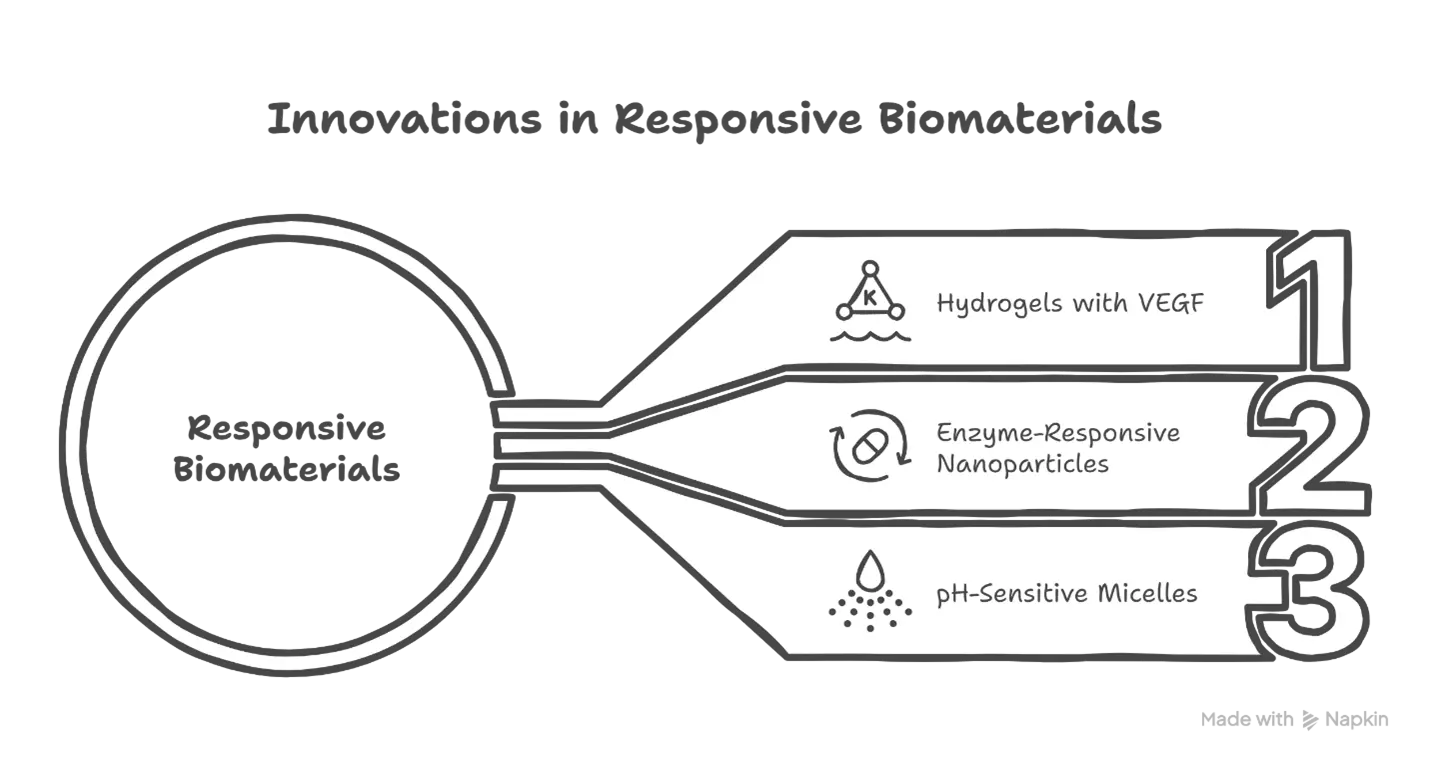
3.2 Growth Factor Release
Another hallmark of responsive biomaterials is their capacity to release growth factors in response to stimuli such as pH changes, enzymes, or mechanical stress. This targeted delivery enhances tissue regeneration while minimizing side effects. Innovations in this domain include:
- Hydrogels embedded with VEGF (vascular endothelial growth factor) that release the factor in hypoxic environments to promote angiogenesis.
- Enzyme-responsive nanoparticles that deliver BMP-2 (bone morphogenetic protein) specifically in bone injury sites.
- pH-sensitive micelles for controlled release in inflamed tissues.
4. Applications in Tissue Growth
5. Challenges and Future Directions
Despite their advantages, responsive biomaterials face several challenges:
- Achieving precise control over degradation and release kinetics
- Balancing mechanical strength with bioactivity
- Scaling production while maintaining reproducibility and biocompatibility Future research should focus on integrating multiple stimuli-responsiveness, real-time monitoring capabilities, and patient-specific customization to further enhance therapeutic outcomes.

