Introduction
Stem cell research has significantly advanced over the past few decades, leading to groundbreaking possibilities in regenerative medicine. Traditional stem cell sources, such as embryonic stem cells, present ethical challenges and limitations. The emergence of iPSCs, derived from adult somatic cells, has overcome many of these obstacles. This paper will explore the potential of iPSCs to differentiate into various tissues, offering the possibility of repairing or replacing damaged tissues and organs.
Background:
Induced Pluripotent Stem Cells (iPSCs) were first developed in 2006 by Shinya Yamanaka, who demonstrated that adult somatic cells could be reprogrammed to a pluripotent state. iPSCs share similar properties to embryonic stem cells, including the ability to differentiate into all three germ layers, making them versatile for various tissue engineering applications. Their use bypasses the ethical concerns associated with embryonic stem cells. Despite their potential, challenges such as tumorigenicity, immune rejection, and the need for efficient differentiation protocols remain.
Methods:
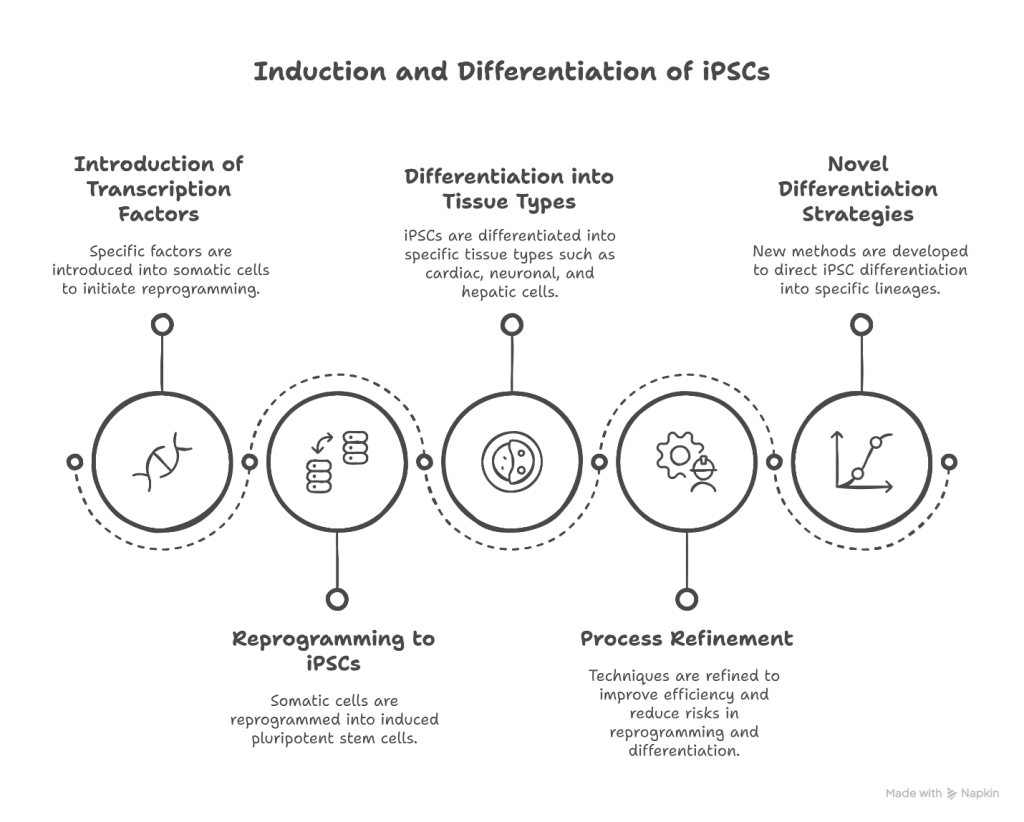
Role of iPCsin Tissue Bioengineering
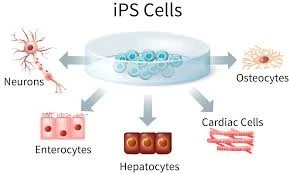
iPCs play a pivotal role in the field of tissue bioengineering by serving as a source of patient-specific cells capable of differentiating into various tissue types. These cells can be integrated with biomaterial scaffolds—such as natural hydrogels, synthetic polymers, or decellularized extracellular matrices—to develop functional tissue constructs. Recent advancements have enabled the differentiation of iPSCs into specialized cells like cardiomyocytes, neurons, and hepatocytes, which are then seeded onto 3D scaffolds to mimic the architecture and microenvironment of native tissues. Additionally, the integration of iPSCs with 3D bioprinting technologies has facilitated the creation of complex tissue constructs, enhancing the reproducibility and scalability of engineered tissues for therapeutic use.
Future Applications and Perspectives
As the technology surrounding iPSCs and tissue engineering continues to evolve, future applications are expected to expand into the fabrication of whole organs, including bioengineered kidneys, lungs, and hearts. One promising direction is the development of personalized tissue grafts for transplantation, reducing the risk of immune rejection by using autologous iPSCs. Additionally, organ-on-a-chip platforms and disease models derived from patient-specific iPSCs will play a crucial role in drug testing and precision medicine. Advances in gene editing, such as CRISPR-Cas9, may further enhance the therapeutic potential of iPSCs by correcting genetic defects before tissue construction. Despite current challenges—such as tumorigenicity and functional maturation—ongoing research is steadily paving the way toward clinically viable, lab-grown tissues and organs.

